Drug discovery has become increasingly sophisticated and challenging in recent years due to the emergence of various modalities for diverse medical needs. In this environment, the Group urgently needs to pursue drug discovery programs that will progress to the clinical stage.
Under the medium-term business plan “Vision 110 –Stage1–,” we will use a new drug discovery strategy to take on the challenge of drug discovery innovation, with the aim of “strengthening drug discovery capability to create high-value new drugs that meet medical needs.”
By combining promising drug discovery targets identified from disease research with the latest drug discovery technologies, we will create new drugs with clinical significance.
In addition to small molecule drug discovery, which is our specialty, we will actively utilize nucleic acid drug discovery and new external technologies as new modalities to create new drugs with higher value. For this, we will incorporate superior external technologies with our own technologies and ideas.
We will also improve the expertise of our researchers and broaden their perspectives through collaboration with external organizations. In these and other ways, we will work to develop human resources who will play key roles in realizing the long-term vision “Vision 110.” In fiscal 2023, we will strive to initiate and promote high-value research projects and strengthen our drug discovery technologies.
At the same time, we will promote multiple development projects based on our development strategy to maximize value and achieve new milestones.
Changing environment(internal and external)
- Increasing sophistication and difficulty of drug discovery
- Diversification and complexity of drug discovery modalities and basic technologies
- Evolution and proliferation of digital technologies
- Expansion of the healthcare field with the entry of companies from different industries
- Growing concern about social security costs and moves to curb them
Opportunities
- Development of basic research technologies to increase drug discovery research opportunities
- Acceleration of research through activation of open innovation
- Use of big data and AI to streamline R&D
- Increase in new treatment options thanks to digital technologies
- Increasing importance of drugs with superior medical economy
Risks
- Increased use of AI in drug discovery making small molecule drug discovery more efficient (through major cost reductions and shorter development periods), which could weaken our drug discovery advantage
- Megapharmaceutical manufacturers deploying digital technology to increase speed of development
- Further rise in development costs due to stricter clinical trials and stricter approval of new drugs
- Market contraction due to reform of drug pricing system and its impact on business viability
Medium-term business plan
Vision 110 –Stage1– initiatives
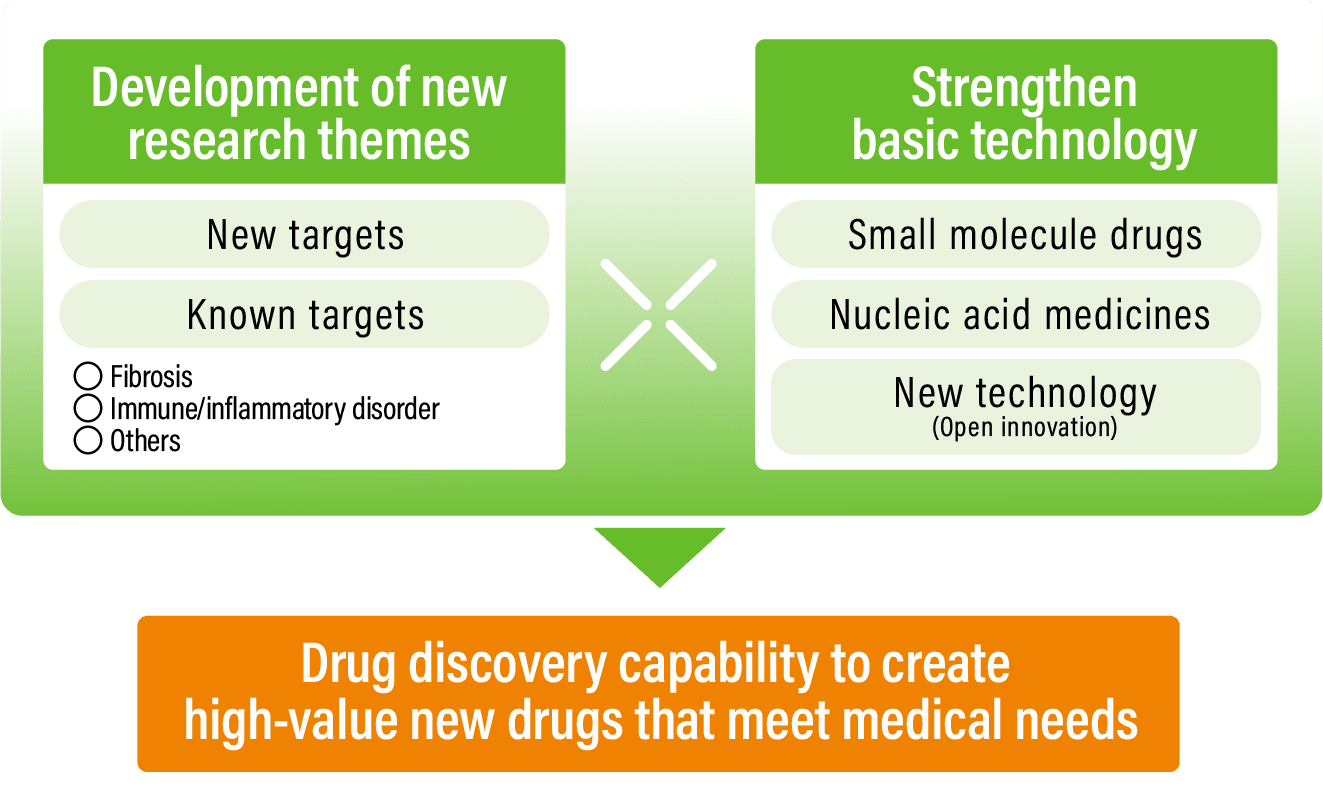
Business strategyStrengthening drug discovery capability to create high-value new drugs that meet medical needs
Pursue drug innovation through new drug discovery strategies
- Engage in drug discovery using novel technologies for existing treatments that have issues, in addition to drug discovery for diseases where drug contribution is low
- Combine drug discovery technologies and disease research to create new high-value drugs
- Drug discovery technologies: Deploy nucleic acid drug discovery and new external technologies, in addition to small molecule drug discovery
- Disease research: Focus on fibrosis, immune and inflammatory disorders, and other diseases
Initiatives under the medium-term business plan
Pursue drug discovery innovation through new drug discovery strategies.
New drug discovery strategies
In the Group’s core business of new drugs, the continuous creation of new drugs is difficult. To address this issue, we need to pursue drug discovery programs and formulate exit strategies for progressing to clinical trials, and we have strengthened our organizational functions to this end.
By combining drug discovery technologies and disease research (drug discovery targets), we will take on the challenge of “drug discovery innovation” to create new value. In the past, we focused on diseases where drug contribution is low (unmet medical needs) and concentrated on drug discovery for those diseases. Under Vision 110 –Stage1–, however, we will work on drug discovery that creates clinical significance by deploying new technologies for existing treatments that have issues.
We have identified fibrosis, immune and inflammatory disorders, and other diseases as our focus areas for drug discovery research and are working to create and promote new drug discovery programs by advancing disease research in these areas.
Strengthen collaboration with external organizations and establish research projects
In research on fibrosis, we collaborated with the Department of Drug Discovery for Lung Diseases at the Kyoto University Graduate School of Medicine to build a unique pulmonary fibrosis model and launch a new research project focusing on triggers of fibrosis.
We are also collaborating with academia to strengthen our target verification technology. In other areas, we are exploring opportunities for joint research with domestic companies, international companies, and academia. In drug discovery technology, meanwhile, we will build a foundation in nucleic acid drug discovery and utilize external technologies, in addition to reinforcing our capabilities in small molecule drug discovery, one of our strengths. By incorporating not only our own technologies and ideas but also superior external technologies, we will create new drugs with higher value. Pursuing value creation, we will select and focus on research projects while formulating and verifying exit strategies.
In the initial exploratory research stage, our drug discovery activities emphasize target therapeutic profiles (TTPs) and scientific approaches to them. After optimization research into leading compounds, we base our decisions on whether to move forward on target product profiles (TPPs).
Expand development pipeline through in-licensing and formulate development and medical strategies to maximize value
We will strengthen cooperation with relevant departments to speed up evaluation and acquisition of candidates for in-licensing. For development candidates, we will formulate highly distinctive development strategies while being always attentive to novel clinical evaluation methods and therapeutic strategies.
Regarding the licensing agreement concluded in January 2020 with U.S.-based aTyr Pharma, Inc. for KRP-R120: efzofitimod (genetic recombination), a fusion protein formulation, we started Phase III multiregional clinical trials in September 2022.
Those trials are progressing well. In November 2022, we concluded an agreement with SUSMED, Inc. for the joint development and marketing of KRP-DT123, a therapeutic application in the field of otolaryngology, a priority area for Kyorin. In this field, we contribute to medical care by providing patients new treatment options based on approaches different from pharmaceuticals. We have started specific clinical research using KRP-DT123 to treat tinnitus in fiscal 2023.
As we expand our development pipeline, including for in-licensed products, we expect to diversify modalities and pursue global development. To address them, we will develop our own strategies and strengthen our regulatory function.