To continue as a company that contributes broadly to people’s health, we are constantly pursuing the challenge of creating high-value new drugs that meet medical needs.
Therefore, in tandem with our in-house drug discovery innovation, we are expanding the development pipeline through in-licensing from external parties.
We are also working to stabilize existing business alliances and create new businesses through out-licensing to secure earnings, with the aim of supporting the continuous growth of Kyorin’s new drugs business.
We have already acquired four new in-licensed products during the period covered by the medium-term business plan “Vision 110 –Stage1–” and are aiming to acquire at least two in-licensed products during fiscal 2025 to solidify our business base.
Changing environment
- Advances in digital transformation in medicine
- Increasing sophistication, difficulty, and higher costs of drug discovery
- Diversification and complexity of drug discovery modalities and basic technologies
- Depleted development pipeline
Opportunities
- Expansion of technological innovation through open innovation
- Increase in opportunities to collaborate with partners from different industries
- Significant increase in capital and human resources
Risks
- Surging investments in in-licensing contracts
- Intensified competition for acquisition of in licensed products
- Increased difficulty in development
Medium-term business plan
Vision 110 –Stage1– initiatives
Business strategyExpanding development pipeline through in-licensing
Significantly strengthen our ability to acquire in-licensed products
- Expand modalities and disease areas targeted for in-licensing and pursue wide-ranging in-licensing activities
- Increase in-licensing investments and boost investments in human resources
Promote development of digital therapeutics (DTx)
- Develop a therapeutic application in the field of otolaryngology
- Disease research: Focus on areas including pain and autoimmune disorders
Search and evaluation through organizational reforms and deployment of human resources
Strengthening our in-licensing search and evaluation is essential to achieve our “Stage1” target of acquiring six in-licensed products. We are always considering multiple projects simultaneously, making it important for relevant departments to work closely to proceed with speedy and accurate evaluations.
We have therefore established the Licensing Department (for licensing activities) and the Alliance Department (for contract negotiations and management of alliances) within the Business Development Headquarters, creating a “one-stop” in-licensing structure for the entire process from exploration to evaluation, negotiation of terms and conditions, and conclusion of contracts.
Making full use of this strengthened organization and functionality as well as our human resources, we will strive to maximize both the volume and the quality of our in-licensed product acquisition activities.
Expanding in-licensing target modalities and disease fields
To broaden our development pipeline, we need to expand target modalities and disease fields and pursue in-licensing initiatives in a wide range of areas.
In addition to small molecule drug discovery, we aim to acquire at an early date in-licensed products with viable commercial prospects that will enable us to demonstrate our strengths in new modalities and disease fields outside our priority areas of respiratory, otolaryngology, and urology. With competition to acquire promising new drug candidates intensifying, we will increase our investment in in-licensed product acquisition with an emphasis on both the volume and quality (newness) of development information.
This approach will lead to creating new drugs that contribute to people’s health, and we will tirelessly pursue in licensing activities.
Pursuing proactive partnering activities
The Business Development Headquarters’ Alliance Department and Licensing Department work closely with other relevant departments to develop proactive partnering activities.
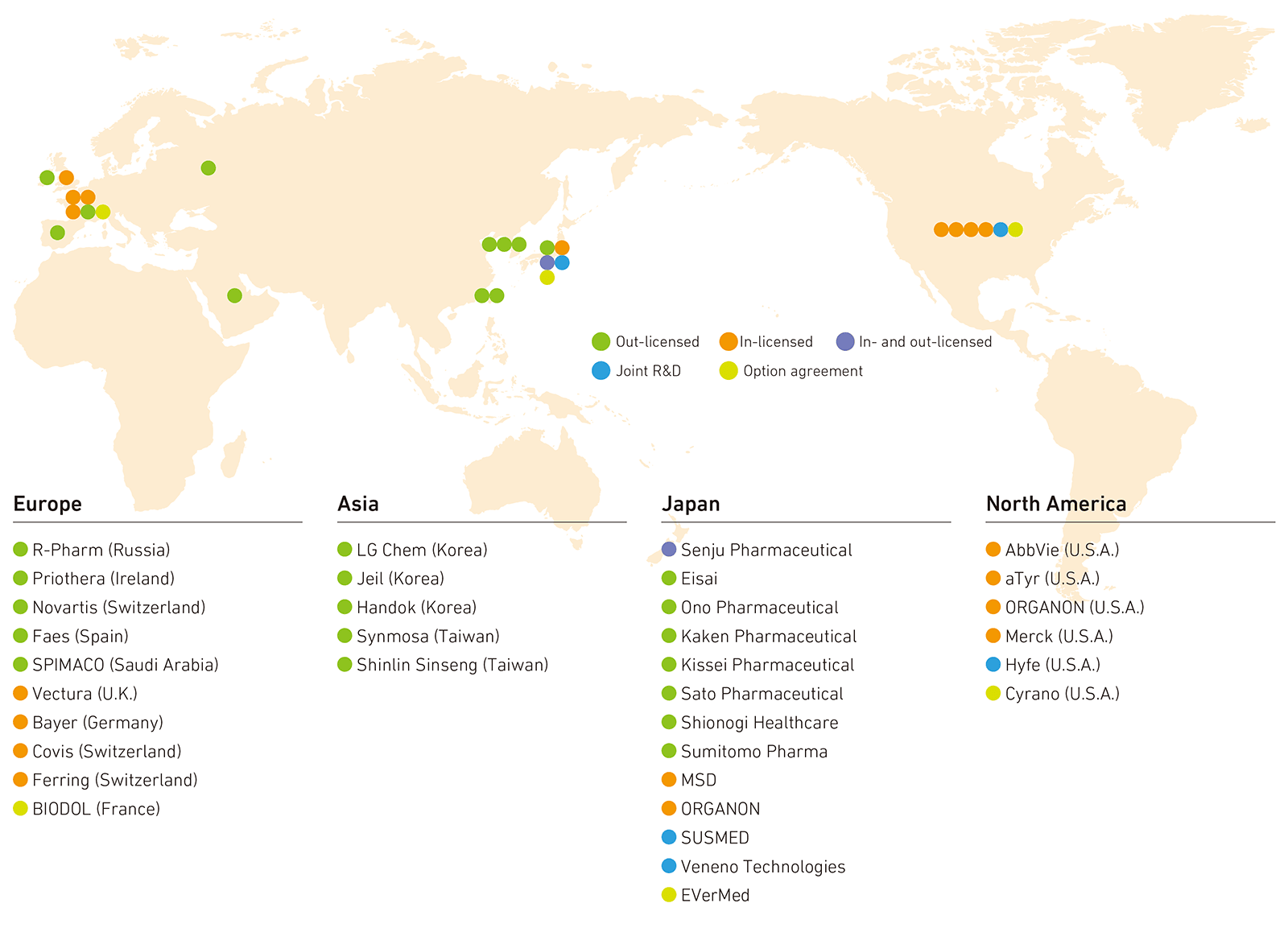
We concluded an agreement in January 2020 with aTyr Pharma of the United States for the interstitial lung disease treatment KRP-R120 and reached an agreement in April 2021 with MSD for the exclusive distribution rights in Japan of the intractable chronic cough treatment Lyfnua (launched in April 2022). We further expanded the development pipeline by concluding a licensing agreement with Bayer of Germany in December 2024 for KRP-S124, a candidate compound for treating obstructive sleep apnea. In addition, in January 2025, we concluded an option agreement with BIODOL Therapeutics of France for the candidate compound for pain treatment BDT272, as well as an option agreement in February 2025 with Cyrano Therapeutics of the United States for CYR-064, a treatment for post-viral loss of smell.
For therapeutic application development, in November 2022, we concluded an agreement with SUSMED to jointly develop and market KRP-DT123, a therapeutic application in otolaryngology, and, in September 2023, began specific clinical research to treat tinnitus. We are engaged in joint development with Hyfe of the United States to develop the chronic cough treatment application KRP-DC125, which uses AI to monitor coughing based on the principles of the non-pharmacological treatment behavioral cough suppression therapy (BCST).
Our existing businesses maintain alliances with several dozen companies in Japan and overseas, as we work to create new businesses through proactive partnering activities.
Promoting global out-licensing activities
We are proactively pursuing out-licensing activities with global companies to maximize the value of our proprietary products. In October 2020, we concluded an agreement to transfer the intellectual property rights of the immunomodulator KRP-203 to Priothera of Ireland; in March 2021, we signed a licensing agreement with Eisai to develop and sell the overactive bladder treatment Vibegron (sales name in Japan: Beova) in four ASEAN countries; and in March 2023, we signed a licensing agreement with Sumitomo Pharma to develop, manufacture, and sell Vibegron in Taiwan and other regions.
We also concluded a global licensing agreement with Novartis of Switzerland in March 2025 for our proprietary KRP-M223. Going forward, we will continue to engage in proactive partnering activities worldwide to quickly roll out our own products in various countries and regions to provide high-value pharmaceutical products that contribute to people’s health.
Partnering with Companies in Japan and Overseas