KYORIN Pharmaceutical Co., Ltd is working proactively to invest pipeline expansion and to conduct clinical trials with the aim of enhancing corporate value.
Strengths
- Expertise, personal connections, and networks for product development in the fields of respiratory, otolaryngology, and urology (development capabilities in designated disease fields)
- Development strategy with cooperation by drug discovery, marketing and strategic planning divisions
Opportunities
- Increase overseas clinical trial and multiregional clinical trials
- Raise precision of test subject selection through development of diagnostic technologies
- Use digital technologies to realize efficient operations
- Use early approval and other systems to provide treatment opportunities more quickly
- Promote development by creating and using an ecosystem and coordination across it with other industries, academic institutions, etc.
Risks
- Significantly higher development costs from stricter standards for clinical trials and new drug approvals
- Increasingly severe effect on business viability from reform of the drug pricing system
Initiatives under the medium-term business plan
Expanding the pipeline to support growth
KYORIN Pharmaceutical, which considers the expansion of the pipeline to support medium-term growth an important management issue, is working to expand the development pipeline in its designated fields (respiratory, otolaryngology, and urology) as well as in infectious disease and in rare and intractable diseases.
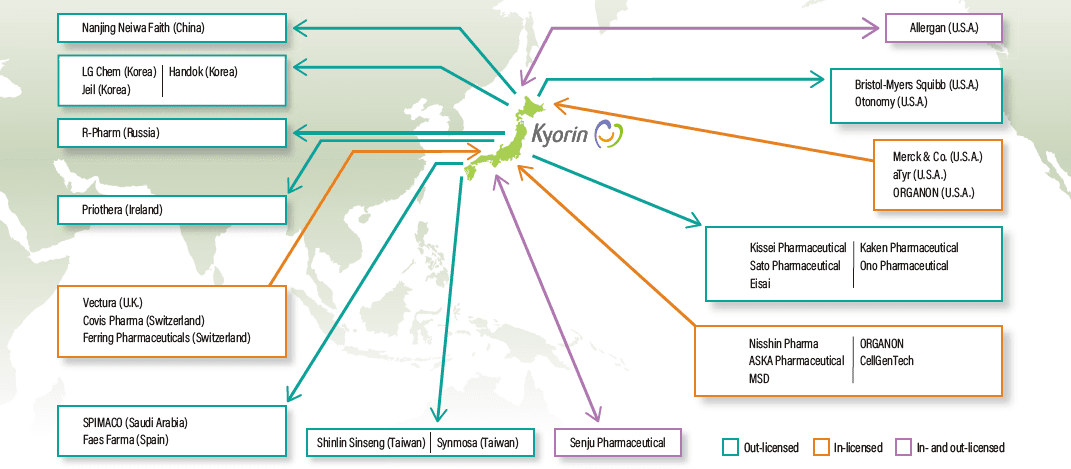
We began Phase 1 clinical trial of KRP-A218, a treatment for rhinovirus that risks becoming aggravated. Phase 1 clinical trial of interstitial lung disease (pulmonary sarcoidosis) treatment KRP-R120 was completed, and entered into the multiregional clinical trials (Phase 3 study) with the out-license, aTyr Pharma.
We also aim to roll out global licensing activities for proprietary compounds at an early date.
Products under development
Promotion of proactive partnering activities
KYORIN Pharmaceutical Co., Ltd. expanded its development pipeline in September 2020 with the conclusion of an agreement with ASKA Pharmaceutical Co., Ltd. for the joint development and sales of benign prostatic hyperplasia treatment AKP-009, and in April 2021 completed and agreement with MSD K.K. for the exclusive distribution rights in Japan of Lyfnua.
In regard to the licensing agreement concluded in January 2020 with aTyr pharma, Inc. for KRP-R120, Phase 3 clinical trial (multiregional clinical trials) has started. We also begun joint development with Lumen Bioscience, Inc. for spirulina genetic protein engineering technology and CellGenTech, Inc. for Fabry disease, and SUSMED, Inc. for the development of digital therapeutics in Otolaryngology field.
Going forward, KYORIN Pharmaceutical Co., Ltd. will continue its proactive partnering activities worldwide, with the aims of expanding the product pipeline to support medium-term growth and establishing a strong presence in our priority fields of respiratory, otolaryngology, and urology.
Promoting global out-licensing to increase overseas earnings
To maximize the value of its proprietary products, KYORIN Pharmaceutical Co., Ltd. is proactively engaged in out licensing activities with global companies. In Octorber 2020, we concluded an agreement for the transfer of the intellectual property rights for the immunomodulator KRP-203 to Irish company Priothera Limited, and in March 2021, a licensing agreement was concluded with Eisai Co., Ltd. related to the development and sales in four ASEAN countries of the overactive bladder treatment Vibegron (salese name in Japan: Beova).
The company also concluded a licensing agreement related to the new quinolone oral antibacterial agent Lascufloxacin (sales name in Japan: Lasvic) with the Chinese company Nanjin Faithe Co., Ltd. in March 2022, granting it exclusive development and commercialization rights in China.
Partnering with Companies in Japan and Overseas
Initiatives toward direct entry into Asia
In tandem with our licensing activities, we conducted market surveys and collected information on Southeast Asia, aiming to lay the foundation for a direct entry into overseas markets in the future, focusing on Asia.
We began selling our multipurpose disinfectant cleaner Rubysta through PT. Meiji Indonesian Pharmaceutical Industries (PT. Meiji Indonesia), a subsidiary of Meiji Seika Pharma Co., Ltd., and also concluded licensing agreement for generic drug manufacturing technologies with the Vietnamese company BinhDinh Pharmaceutical and Medical Equipment JSC, we concluded an agreement giving generic drug sales rights to the Mongolian company Monospharm Trade Co., Ltd. Going forward, we will continue to consider directly entering overseas markets on the basis of locally collected information and move steadily in that direction.


